


Poten Pharmaceuticals has a dedicated pharmaceutical administration service team to provide CMC services and product launch maintenance for domestic and international customers at different stages of clinical trial application (IND/CTA) and clinical trial (P1, P2, P3), new drug marketing application (NDA/MAA), generic drug marketing application (ANDA), including product change, annual report and re registration services< o:p>
It provides the full life cycle service management of API from R&D to production and marketing, communicates well with you from the early stage of R&D, makes forward-looking analysis on technical differences and pharmaceutical policy differences, interacts well in the R&D process in a timely manner, and provides relevant CMC data of CTD data modules II and III and other forms of documents required by customers to meet the needs of different registration regions< o:p>
The members of the drug administration team have rich experience in registration and declaration at home and abroad. As the DMF Holder, the companies they serve have successfully registered/filed and/or obtained approval for nearly 30 products in 5-8 drug administration authorities. The types of applications include IND, NDA and ANDA registration and declaration. They have the experience of successful application for CMC of bulk drugs in the United States, Europe, Japan, China and other markets
Main test equipment for R&D analysis:
|
Name of main equipment |
model |
manufactor |
Number of units |
|
liquid phaseHPLC |
|
|
|
|
liquid phaseUPLC |
|
|
|
|
Gas phase |
|
|
|
|
LC-MS |
|
|
|
Main test equipment for pilot test
|
Name of main equipment |
model |
manufactor |
Number of units |
|
liquid phaseHPLC |
|
|
|
|
liquid phaseUPLC |
|
|
|
|
Gas phase |
|
|
|
|
LC-MS |
|
|
|
|
Ion chromatography |
|
|
|
|
TOC |
|
|
|
|
…… |
|
|
|
Chengda's professional analytical R&D and QC team provides customers with quality research and stability research services for APIs, intermediates and registered raw materials that meet the global IND/NDA declaration requirements. The project experience covers all stages from preclinical to commercial, including method development/optimization, analytical method validation/transfer, physical and chemical properties research, structure confirmation, reference standardization, product release test, packaging compatibility research, stability research Research and formulation of quality standards to meet the needs of customers at different stages of drug development.
From the initial stage of production design to the life cycle management of equipment and facilities, Boten has established and improved its management system, identified and controlled safety risks, improved production efficiency and safety, and ensured the safety of production operations.
Adhering to the concept of "safety comes from design", the company fully identifies and controls safety risks by adopting HAZOP/LOPA risk methods at the design stage, and adopts scientific control measures to improve the intrinsic safety
The company has established a life-cycle management system for equipment and facilities, introduced automatic control systems, closed control equipment and facilities and other engineering control means, and improved inerting and explosion venting systems to meet the needs of production processes and improve production efficiency and safety
The company has established a sound EHS management system, and formulated sound safety production rules and regulations and operating procedures, including process safety, occupational health and environmental protection risk identification, assessment, detection and control procedures to ensure the safety of production operations and the sustainable development of personnel health and the environment
The company selects appropriate employees and provides adequate skill training and safety training to ensure safe production and operation
5 workshops (202/203/206/207/208): already in use
All workshop floors adopt; Three floor three-dimensional design
Reaction Temperature- 78~200oC (stainless steel+ultra-low temperature glass lined kettle)
Maximum Pressure; 50 bar (2000-3000L, stainless steel+Hastelloy)
Total reaction volume:~950000L (50-8000L)
51 stainless steel reactors, 282 glass lined reactors
7 clean areas (Class D)
High-pressure Hydrogenation Reaction
Anhydrous anaerobic reaction
Chiral compound synthesis technology
Cyanide reaction; (Qualified to use 30% sodium cyanide (NaCN) aqueous solution, annual usage> 1000 tons)
Methylation reaction; (Formaldehyde participates in the reaction and dimethyl sulfate participates in the reaction)
Friedel Crafts (Annual usage of aluminum trichloride> 200 tons)
Oxidation reaction; (H2O2, KMnO4……)
Polymerization; (VA044……)
Ultra low temperature reaction; (-78 oC, 1000-2000L, SS+GL;-30oC, 500-1000L, GL)
High temperature reaction; (>200 oC, 500-1000L)
High vacuum distillation system (vacuum degree up to< 20 pa)
High efficiency film evaporation system (solvent can be removed quickly)
Rapid and accurate measurement of reaction heat, exothermic rate, heat accumulation, reaction outgassing rate, total outgassing, etc. in the reaction process
Measure the initial decomposition temperature, decomposition heat, temperature and pressure changes during sample decomposition. Evaluate the thermal stability of the sample
Assess the hazard level of the process
Based on the reaction scale and equipment parameters, evaluate the feeding rate of process amplification, etc
According to the detailed analysis on process validation in ICH-Q7, in the product production process, it is proved that theproduction used for products meets theGMP requirements through systematic and documented evidence of process validation. And the process is stable and reliable,
Process validation process
Process validation documents: process report, method validation report, batch production record master, process validation scheme
Process validation batch generally includes 1 to 2 pre validation batches and 3 process validation batches
Accelerated and long-term stability tests can be conducted according to customer requirements
Define and confirm key quality influencing factors of the process
Study the fate of actual impurities (greater than 0.10%) and potential impurities in the registered starting materials and separated intermediates
Collect information on registered starting materials and separated intermediates from different batch sources
Redeveloping or adjusting the analytical methods of registered starting materials and separation intermediates to ensure that the actual impurities, potential impurities and impurities generated in the subsequent reaction can be well separated
Carry out corresponding addition experiments on actual isomers, potential isomers, analogues and other by-products in the synthesis process of registered starting materials
Collect the experimental data of the study on the fate of impurities in the registered starting materials and separated intermediates, and compare them with the CQA of API to establish the control strategy for the registered starting materials and separated intermediates
Write quality standard report (record the process research and analysis research data of registered starting materials and intermediates in detail)
Route design and development
Evaluate whether the existing route and related steps meet the requirements of amplification, cost, safety and quality control
A doctor chemist with rich experience can suggest new synthetic routes and carry out laboratory exploration of new routes
Route exploration usually takes 2~3 steps/person week. The process chemist has the authority to operate LC-MS, GC-MS and other equipment, so as to quickly open up the route for subsequent process development
Quickly get through and optimize key steps through parallel reaction equipment
Apply new technology to make unacceptable steps acceptable
Rapid handling and production capacity of hazardous chemicals help route development and promote drug R&D progress
Process development and optimization
Fit for purpose process development and control strategy formulation
Completely carry out new drug IND research and support the application work
API process development and synthesis, supporting GLP TOX research and clinical research
Final process development for commercial production and supply
Process optimization to obtain robust process, reduce cost and improve output
High throughput screening and optimization of high cost catalysts
Salt type screening, crystal type screening, and then salt type crystal type characterization, selection and process development
API recrystallization process development and crystallization process optimization
Heterogeneous mass spectrometry analysis, impurity identification, synthesis and index setting
Application of online monitoring technology to guide process development, such as online infrared, online liquid phase, FBRM, etc
Apply QbD principles to guide process development, such as using DoE tools to conduct design space research
Capable of handling highly active compounds (OEL< 0.1 ug/m3)
Process safety test and evaluation (RC1, DSC, TGA, ARC, TSU)
Chemical engineering simulation capability supports process development (such as filtration rate simulation, oxygen content control assessment) and equipment risk assessment of project transfer (such as selection of kettle and paddle type, simulation and application of distillation parameters and azeotropic efficiency)
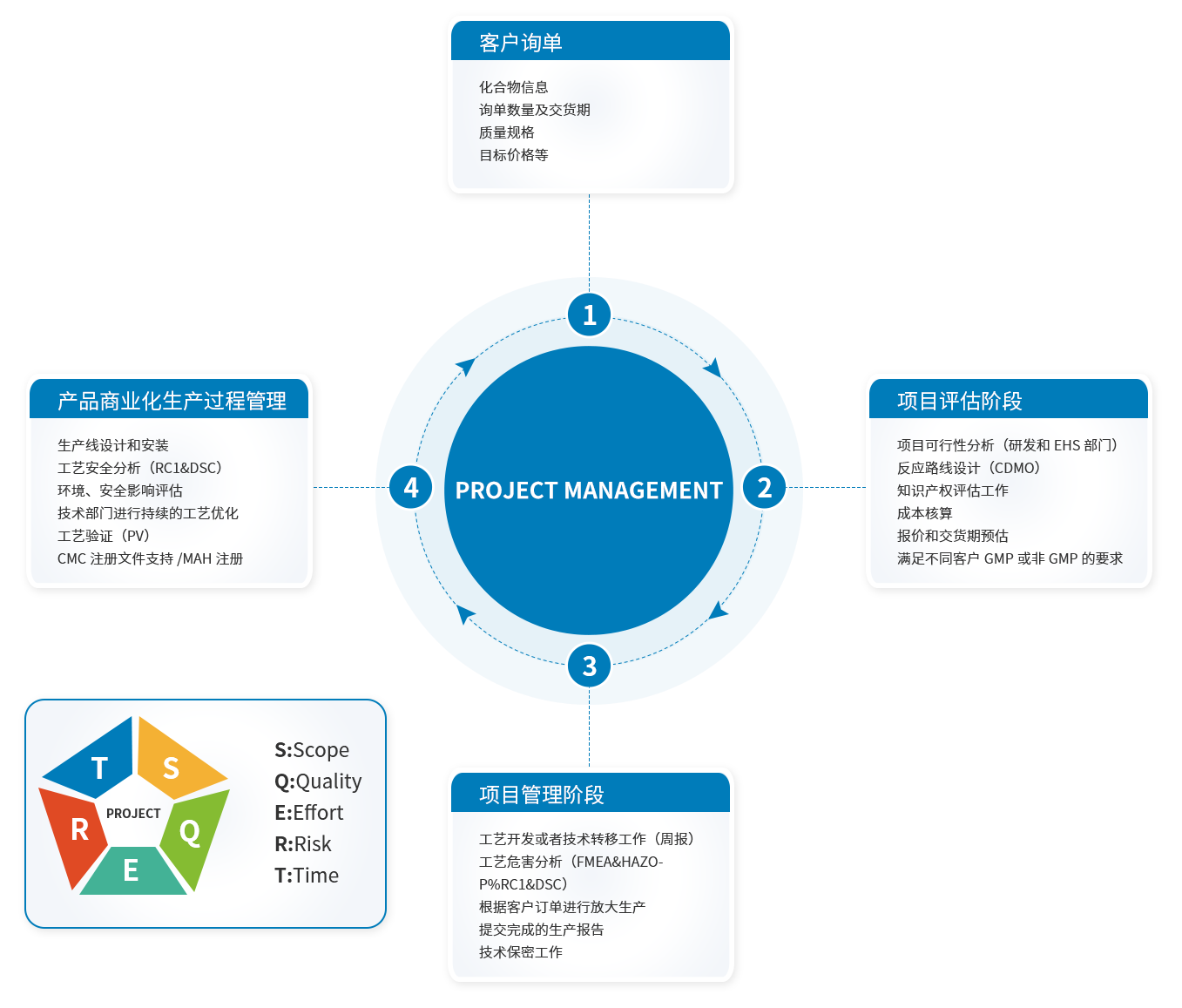
The company's CDMO business model is a pharmaceutical outsourcing service model integrating R&D and production. In a gradual way, the company has carried out the scale up from kilogram to ton, and finally realized the large-scale production of products: in the pilot stage, the company mainly carried out molecular validation, process route exploration and research and development, and completed the stability experiment and kilogram validation of products; In the pilot stage, the company conducts step by step scale-up test, and adjusts and optimizes the process according to the actual situation and relevant problems in the scale-up production process; In the trial production and process validation stage, the company determines the process parameters suitable for the commercial production equipment and forms a relatively complete process procedure. On the basis of all the above R&D and production work, the company finally realizes the commercial batch production and sales of products.
The R&D laboratory covers an area of more than 6300 square meters. It has a small trial synthesis laboratory, a kilogram scale amplification synthesis laboratory, and a R&D analysis laboratory to meet the requirements of R&D, optimization and amplification from milligram, gram to kilogram< br /> The pilot workshop has a building area of 5100M2, and is equipped with anhydrous and oxygen free, high vacuum, high-pressure hydrogenation, reaction in the temperature range of - 100 ℃ to 200 ℃, distillation, rectification and column chromatography separation. It can be used for pilot scale up of 10kg to 100kg. There are two pilot scale clean areas, which can meet the requirements of GMP for product pilot scale up< br /> The company introduces high-end talents from abroad, leads the R&D team, provides customers with R&D and services at different stages, and meets the diversified customization needs of different customers. It has successively established close cooperation with Zhejiang University of Technology, Nanjing University of Technology and other institutions of higher learning, and has in-depth exchanges and cooperation in product development, talent training, resource sharing, etc. It has built the enterprise into a high-tech experimental platform and practice base, accelerated the industrialization process of new technologies, and improved the scientific and technological strength of the enterprise.
